Product
Excellent self-lubricating nature, fatigue behavior, and oil resistance. It is used in gears, bearings, automotive interiors and medical parts.
For inquiries regarding SDS and various chemical substance investigations, please make your request through your purchasing route, such as via a trading company.
We appreciate your understanding and cooperation.

Materials such as POM are used as parts for a variety of medical and healthcare products.
They have the advantage of being lighter and offering a higher degree of freedom in shaping compared to metals and glass.
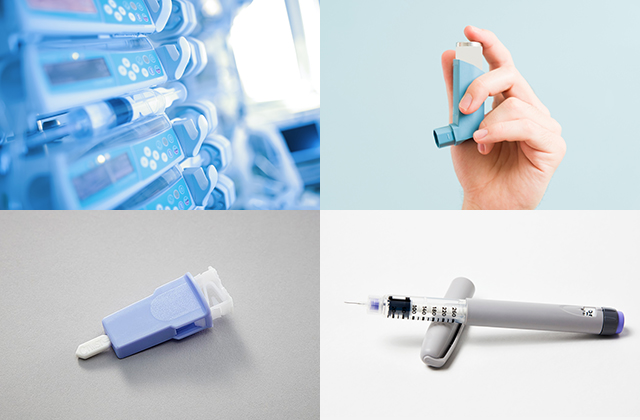
Asahi Kasei recommends our TENAC™ polyacetal (POM) resins as material for medical devices.
Polyacetal resin (Polyoxymethylene, abbreviated as POM) possesses properties ideal for medical device applications, including strength, rigidity, impact resistance, low wear, low friction, resilience, and creep resistance. Especially in various mechanical components, smooth and quiet operation can be expected.
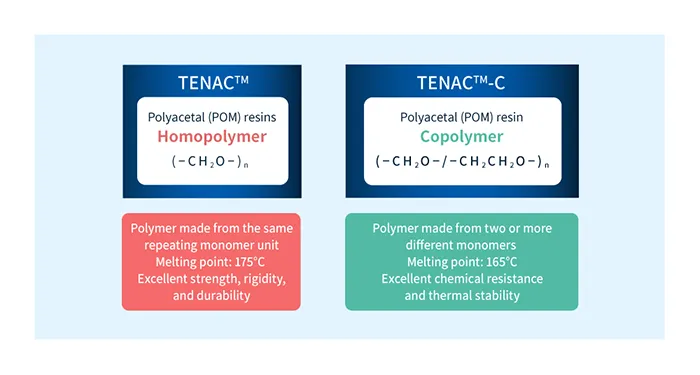
POM is primarily a thermoplastic resin composed of the structure (-CH2O-).
POM resins come in two varieties: homopolymers consisting only of the structure (-CH2O-) which excel in strength and rigidity, and copolymers that include some (-CH2CH2O-) units, which have superior thermal stability.
Asahi Kasei manufactures both the homopolymer (TENAC™) and copolymer (TENAC™ -C) POM resin from the polymerization stage onwards, enabling us to supply the products according to the application.
TENAC™ is a POM resin for medical devices that has been adopted for lancets, which require good moldability and good puncture performance (spring characteristics), and infusion pump guides, which require high friction and abrasion resistance.
TENAC™ Q5010 has been added to our lineup as a new POM resin for medical devices.
TENAC™ Medical POM Q5010 is a medium viscosity homopolymer.
Medical grade POM homopolymer TENAC™ Q5010 Compliance with regulations and standards
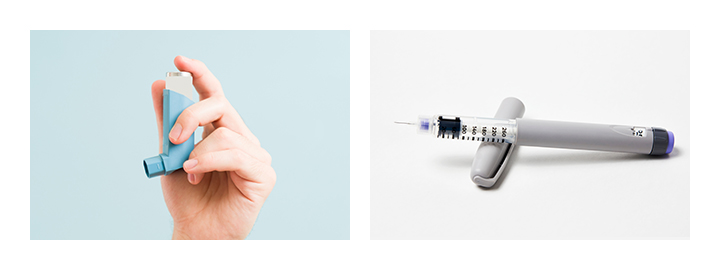
Potential applications for medical POM homopolymer TENAC™ Q5010 include mechanical parts, valve parts and gears in insulin pens and asthma inhalers.
*Please do not use for the following purposes: parts for medical containers and packaging materials that come into contact with the body, mucous membranes, body fluids, or medicines; containers, packaging, utensils, and parts that come into contact with food, drinking water, etc.
Please feel free to contact us with any questions about our products or technologies or to request samples.
We will introduce Asahi Kasei 's engineering plastic products and technologies in more detail.
We regularly deliver product and industry information to help you gather information.